Use group theory to obtain a qualitative MO energy level diagram for the octahedral complex [CrCl6]3-.
First you will need to consider which Cr3+ orbitals may be used in bonding.
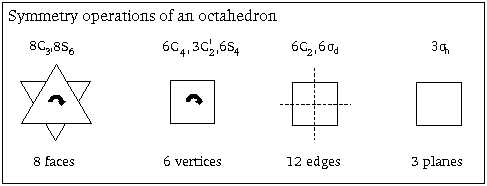
iii) Select an appropriate basis to obtain a reducible representation. Reducing this will give irreducible representations (i.e. symmetries) for the orbitals in the basis.
It is not strictly necessary to know which orbitals on the Cl- ligands are involved: we can instead use six arrows, one pointing from each Cl- towards the central metal atom, to represent σ-bonding orbitals on the chloride ligands and act as a basis for our reducible representation.
 |
Click for larger image |
Click for larger image |
Constructing the molecular orbital diagram - All the required information has been determined above (spectroscopic data can be used to assist in determining the relative energies of the bonding molecular orbitals in [CrCl6]3-).
Comments:
|
| Previous (Exercise 5) | Return to top of page |