| Find the number and symmetries of the Raman and IR active vibrations of the fumarate ion (shown). | 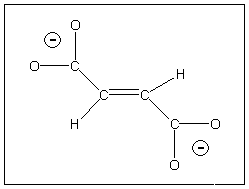
|
- Determine the point group (The fumarate ion is C2h)and using the basis of three arrows (along x, y and z directions) on each atom, obtain a representation.
- Reduce the representation
- Remove the irreducible representations that correspond to translation of the molecule (labelled x, y and z in the character table) and rotation of the molecule (Rx, Ry and Rz in the character table) the remaining irreducible representations correspond to vibrational modes.
C2h E C2 i σh Ag 1 1 1 1 Rz x2+y2,z2 Bg 1 -1 1 -1 Rx,Ry xz,yz Au 1 1 -1 -1 z Bu 1 -1 -1 1 x,y