| Let's now consider Sn where n is odd. For the BF3 molecule, for instance, we might think that S3 ≡ C3, but this only appears to be the case because the molecule is planar. In fact, S3 and C3 are not equivalent and need to be specified separately. |
Click for larger image | |
| In the eclipsed conformation, ethane has an S3 axis. Using the images in the table below, click on the H atom in the product which should be coloured red for each operation performed about the S3 axis in C2H6. |
| Operations | Clickable Image | Corresponding Answer |
|---|---|---|
| S3 |  | |
| S32 | 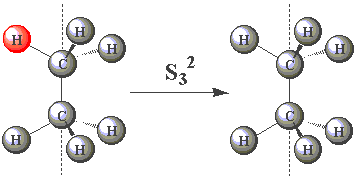 | |
| S33 | 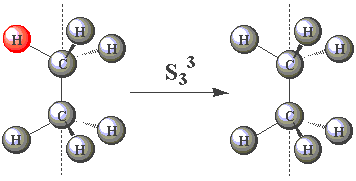 |
Note that the S3 operation is equivalent to neither C3 nor σ.
Using the S3 operations, we must rotate the ethane molecule through a total of 720° before it ends up where it started.
Using the images in the table below, click on the H atom in the product which should be coloured red for each further operation performed about the S3 axis in C2H6.
| Operations | Clickable Image | Corresponding Answer |
|---|---|---|
| S34 | 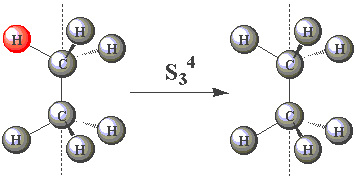 | |
| S35 | 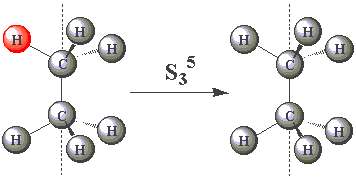 | |
| S36 |  |
Fill in the table below for those S3 operations you think have equivalent symmetry operations generated by other symmetry elements of ethane. Hence, check all the independent symmetry operations we should assign to S3.
(Note — There maybe more than one equivalent symmetry operation for a given S3 operation, if so enter the operations in alphabetical order separated by a comma i.e. a,b )
| Previous (Exercise 2) | Return to top of page | Next (Exercise 4) |